Walking down the toiletries and disinfectants aisle of the store, you remember you need something. You see the alcohol section. Perfect! You were running out. But, wouldn’t you know it, you still have to decide between ethyl alcohol and isopropyl alcohol.
They are both alcohol. As you can see, each brand even offers both variations. Seeing them side to side, you might wonder which is more effective for you and your needs.
In this article, you’ll learn about the differences between ethyl and isopropyl alcohol. Though, let’s start with what ethyl or isopropyl alcohol is anyway?
Ethyl and Isopropyl Alcohol

You might already be familiar with ethyl or isopropyl alcohol. By randomly picking up a bottle, it’s always going to be either isopropyl or ethyl on the bottle. If you’re having trouble deciding, check the definitions below:
What is Ethyl Alcohol?
Ethyl alcohol is the alcohol that might already be in your liquor cabinet. Made from cereal and grains, it’s used in alcoholic beverages, like wine and beer. Though, don’t take out the whiskey to clean just yet.
The ethyl alcohol specifically for disinfecting is denatured. Alcohol is denatured by including additives so that it’s unfit for consumption anymore. They give ethanol a rather unpleasant taste, discouraging people from tasting it. It’s a good thing too, since drinking denatured ethyl alcohol will make you sick.
What is Isopropyl Alcohol?
Isopropyl alcohol is the alcohol commonly used in hand sanitisers, like methanol. It’s mixed in a lot of mass-produced disinfecting and cleaning products. It could also be found in aftershaves and other cosmetics. You can also use certain concentrations of this alcohol by itself as well.
As a rule, rubbing alcohol is unsafe to drink, much like ethyl alcohol once it’s denatured.
The Similarities Between Ethyl and Isopropyl Alcohol

After knowing about what ethyl or isopropyl alcohol is, you can see that they are alike in some ways already. Here are more of their similarities below:
- Both of these alcohols move similarly when breaking down bacteria.
- They are flammable and can evaporate quite easily.
- These two are colourless in nature and soluble in water.
- At room temperature, they do stay in liquid form.
- Ethyl or isopropyl alcohol can defeat most common bacteria like Staphylococcus Aureus, salmonella, and E. Coli.
- Other resistant bacteria they can protect you from are Staphylococcus aureus (MRSA) and Mycobacterium tuberculosis.
Ethyl or Isopropyl Alcohol?

Now that you’ve seen the definitions and similarities of both alcohols, it’s time to look over their differences. Hopefully, the following points will help you decide which is best for you and your family.
1. Chemical Structure
Isopropyl and ethyl alcohol are pretty similar, but they still show differences chemically. Their distinctions in chemical structures give you a deeper insight into how they work.
Ethyl Alcohol
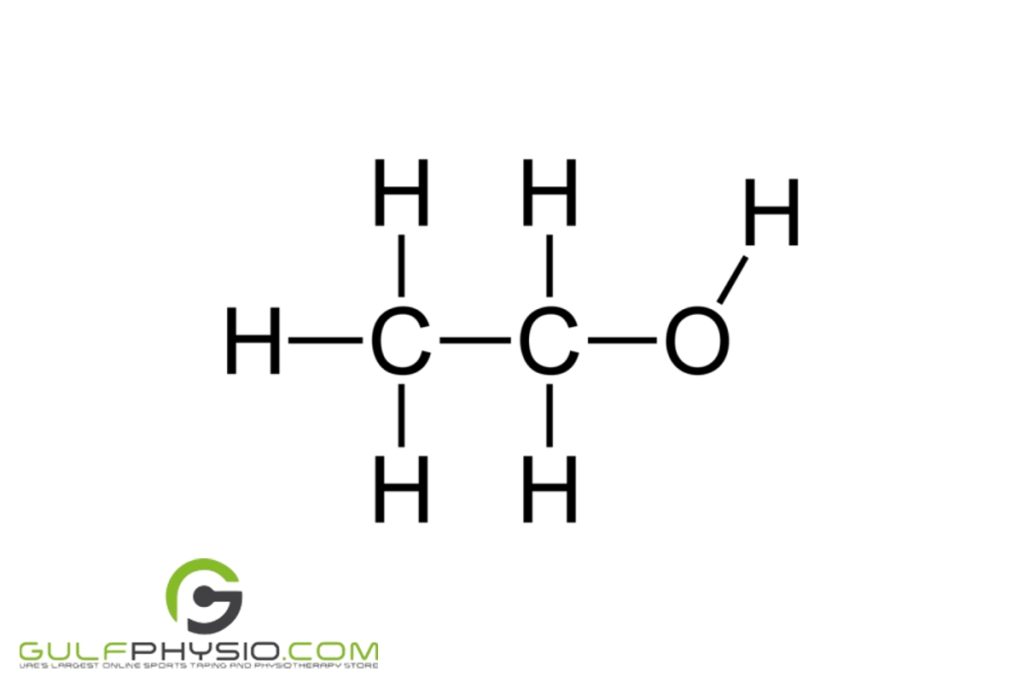
Denatured ethyl alcohol has the chemical formula of C2H6O. Due to its higher octane number, it’s commonly used in renewable gasoline. You can also call this type of alcohol by its other names, which are ethanol or grain alcohol.
Isopropyl Alcohol
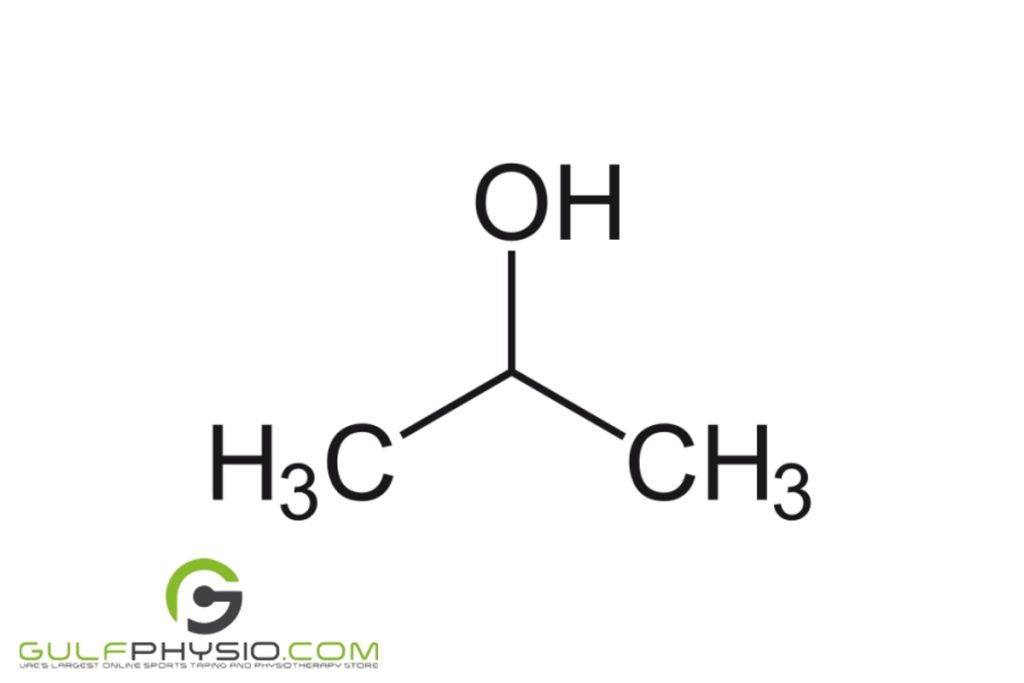
Isopropyl alcohol is a bit different. Its chemical structure is C3H8O. As you can see, it has two more hydrogen atoms and one more carbon atom than ethyl alcohol. Other names for this particular alcohol are isopropanol, IPA, and just regular rubbing alcohol.
2. Physical Characteristics
Both variations of alcohol do look the same. You have to look at other aspects of each solution to know which works best in a given situation.
Ethyl Alcohol
They do have some similarities, apart from their differences in melting points. The main difference between ethyl alcohol and isopropyl alcohol is whether or not you can drink them or not. Ethyl alcohol is the only one safe enough to drink. Granted that it didn’t go through the denaturing process yet.
Isopropyl Alcohol
In any form, you shouldn’t consume any amount of isopropyl alcohol. For factors such as higher boiling and melting points, isopropyl alcohol takes the cake. Its scent is also a bit stronger than ethyl alcohol.
3. Contact Time on Skin
It’s safe to say that people have been using isopropyl and ethyl alcohol for a while now. You can see that it’s safe to use on the skin.
However, these alcohols do clash on their wet/contact time, or the time it takes for it to eliminate bacteria. A shorter wet time indicates that the disinfectant is more powerful because it can kill pathogens faster. Though, keep in mind, if it’s too fast, it won’t have enough time either. The majority of first aid kits contain disinfectants in bottles, or in sprays. Mind you, certain sprays such as those in pressurized canisters probably don’t contain alcohol but instead contain another antiseptic such as chlorhexidine. Take a look at this blue savoy spray for example.
Ethyl Alcohol
Ethyl alcohol is shown to be the best bet for your skin in general. It promotes less skin damage, even when used frequently. It’s slightly thicker than isopropyl alcohol, making your skin dry faster. It doesn’t necessarily give you the best feeling when it does that.
Isopropyl Alcohol
For unbroken skin, it’s best to use isopropyl alcohol. It’s safer since it’s less irritating in small amounts. If you do end up using a lot, the solution could make your skin itch, crack, and red.
Additionally, the slightly more watery consistency of isopropyl alcohol. Because of this, the solution evaporates quickly on the skin.
Are ethyl and isopropyl alcohol bad for the skin?
Ethyl alcohol and isopropyl alcohol are used as disinfectants and antiseptics, which means both are effective at killing germs when used in concentrations over 60 percent. However, there are some studies that ethyl alcohol is less damaging to the skin. Ethyl alcohol is safe for consumption in small amounts.
Meanwhile, exposure to high concentrations of ethanol vapors may irritate the eyes, skin, and respiratory tract, and lack of coordination. It can also result in dizziness, shallow respiration, unconsciousness, and death. Ethanol is harmful by ingestion, inhalation, or skin absorption.
4. Preferred Concentrations
For alcohols, certain concentrations have been proven to be the most effective. If the concentration is too low, it won’t be strong enough to do its job.
On the other hand, if the concentration is too high, it won’t be any good either. Instead of protecting your skin, it’ll protect the germs themselves. For the best results, here are the preferred concentrations for either ethyl or isopropyl alcohol below:
Ethyl Alcohol
Ethyl alcohol is more effective when in concentrations of at least 60% and above, as recommended by the CDC. With the best concentration possible, it can take out a variety of viruses covered in this lipid bilayer, or enveloped viruses.
While we carry 99% ethanol, it’s mostly for industrial purposes. In this case, it’s not for personal everyday use. You won’t find them in regular first aid kits. It’s used mostly for sanitizing surfaces before application.
Isopropyl Alcohol
As for isopropyl alcohol, it’s best to have a 70% concentration, which we carry on our website. When the concentration goes to about 90%, the solution will give the bacteria a coagulated barrier. It’ll end up making the pathogens stronger and harder to kill.
5. Antimicrobial Capabilities
Now, for the meat of the debate. People buy ethyl or isopropyl alcohol as a disinfectant, after all. The following are the various pathogens each alcohol can protect you from. Let’s start with the tough-to-tackle viruses.
Ethyl Alcohol
Ethyl alcohol, as stated in the previous point, is efficient against enveloped viruses. Well, it too can protect you from some non-enveloped viruses like:
- Adenovirus type 5
- Poliovirus type 1
- Murine norovirus
- Astrovirus
- Enterovirus
- Rhinovirus
- Echovirus
- Rotaviruses
A 70% solution of ethyl alcohol has antifungal properties as well. It can eliminate fungi such as:
- Blastomyces dermatitidis
- Coccidiodes immitis
- Cryptococcus neoformans
- Histoplasma capsulatum
Isopropyl Alcohol
Much like ethyl alcohol, isopropyl alcohol can be used against enveloped viruses like the following:
- Herpes virus
- Hepatitis B virus
- Influenza virus
- Vaccinia virus
This alcohol does have a slight edge over ethyl alcohol with some bacteria. It’s more effective against bacteria such as S. aureus and E. coli. Even a 20% solution of isopropyl alcohol can kill off Acanthamoeba culbertsoni.
How Does Alcohol Work as a Disinfectant and Antiseptic?

Ethyl and isopropyl alcohol can eliminate a variety of viruses and bacteria, based on a study conducted in 2020. However, how they do that is quite fascinating.
It’s been recorded that ethyl and isopropyl alcohol do relatively the same thing. They clean up surfaces through the process of denaturation. These organic compounds disintegrate the lipids and proteins that make up most germs. In turn, these cells won’t be able to live for much longer.
Alternative Uses of Ethyl and Isopropyl Alcohol

Apart from disinfecting surfaces, you’ll be surprised about the diverse uses of both alcohols. Listed below are just a couple of the rather unique applications for ethyl and isopropyl alcohol. They are used for:
- Prepping skin for a tattoo or piercing
- Preparing cars for car wraps
- Cleaning electronics like contact pins
- Evaporating excess water from ears
- Detaching stubborn stickers and decals
- Being a quick-fix deodorant
- Sanitizing jewellery and makeup brushes
Other Uses of Ethyl Alcohol
Ethyl alcohol is present in a bunch of your household cosmetics and perfumes. Even protecting your cars just got a little bit easier with it. If you’re looking for an easy but affordable way to customise and safeguard your vehicle, car wrapping is an option. Car wrapping essentially gives you that extra layer of style and defence. Any big bump will only be a ding or a scratch.
Ethyl alcohol comes into play by helping prepare your car for the wrapping itself. After a good wash with soap and water, a 90% ethyl alcohol solution is applied. The alcohol creates the ultimate clean exterior for the wrap to cling to the surface of your vehicle.
Other Uses of Isopropyl Alcohol
Rubbing alcohol is the popular choice for a surprising number of household cleaning. It’s a good addition to your home since it removes stains from your clothes and walls. This alcohol even keeps your shoes fresher than any other product around.
You might not know its cleaning capabilities on your appliances and gadgets. It does its magic on chrome, sinks, and stainless steel. Simply combine one (1) part water with two (2) parts isopropyl alcohol in a convenient spray bottle. You can spritz it directly on your appliances like your toaster and phone. It’ll leave any surface decontaminated and streak-free.
In Conclusion
Either ethyl or isopropyl alcohol is a popular disinfectant. Both of them. They break down bacteria in the same way. They’re soluble, colourless, and volatile. Even when they’re practically the same, appearance-wise, they do some things differently.
The difference in their chemical structures makes them used in other ways, like for aftershaves and gasoline. There are certain ethyl alcohols that you can drink. Before the denaturation process, they’re perfectly safe to consume. In terms of isopropyl alcohol, it’s dangerous in any form.
On your skin, each alcohol acts in its own way. Ethyl alcohol won’t damage the skin that much if used often. Its viscosity makes it dry quicker though. If you want the safer option, isopropyl alcohol works well on unbroken sin.
For concentration measurements, it varies. Isopropyl alcohol is normally used at a 70% concentration. Ethyl alcohol concentrations should start at 60%. Each concentration is used for a specific range of germs, and even fungi.